Projects
By making pharmacogenomic information prompt, accessible, and understandable for physicians, The Center for Personalized Therapeutics leads the way in translating genomic discovery to improve care for patients.
“Translating genomic discovery to improve care for patients.”
The Center embarked on its first project in 2011, the 1200 Patients Project, which uses a preemptive medical system model that reduces the use of high risk medications. Later, CPT established the ACCOuNT Project, Impress Trial, PhOCus Trial.
1200 Patients Project
Piloting the use of pharmaco-genomics in routine medical care.
ACCOuNT Project
Examining pharmacogenomic implementation in the context of minority health disparities.
ImpreSS Trial
Implementing pharmacogenomic testing in anesthesia and perioperative medicine.
PhOCus Trial
Studying pharmacogenomic testing in Oncology care.
Personalized Therapeutics Clinic
Expanding access to personalized therapeutic consultations to high risk patients.
CHABLIS Project
Longitudinal research to promote healthy aging behaviors.
Diversity Project
Dr. Saulsberry 📌
Pharmacoeconomics
Dr. Ratain 📌
The 1200 Patients Project
The 1200 Patients Project aims to develop a new model for personalized medical care through preemptive pharmacogenomics.
Enrolled patients provide a single blood sample for analysis, which is genotyped by an in-house CLIA-certified laboratory. Patients’ genetic information is compared to a database of well-substantiated pharmacogenomic research compiled by the 1200 Patients Project team, which is constantly updated to reflect the most current research available. This database is used to determine whether it is likely that a patient will respond positively or negatively to a medication. With patient permission, these results are delivered to enrolled physicians at the University of Chicago through an online portal, termed the Genomic Prescribing System (GPS).
The GPS is designed to be convenient to use, with clear and consistent images and wording, so that pharmacogenomic information can be easily integrated into clinicians’ workflow. Ultimately, the GPS enables physicians to make patient-specific treatment decisions, improving patient outcomes.

“Finding the Right Medication and Dose”
Adverse reactions to medications are one of the leading causes of death in the United States and many patients take medications that are not effective for them. The information distributed to providers in this study could allow them to identify which patients would experience negative side effects from medications, and to determine which medications would be the most effective for each patient. Pharmacogenomic information can also be factored into dosing algorithms, so that the ideal dose for each patient can be most accurately determined, eliminating the trial-and-error process that is frequently required.
Providers enrolled
Patients recruited
ACCOuNT Project
African American Cardiovascular pharmacogenetics CONsorTium (ACCOuNT) is an NIH-supported, multi-institutional group of physicians, researchers, and patients whose goal is to reduce disparity in precision medicine, particularly for African-Americans.
The ACCOuNT Translational project aims to explore the feasibility of implementing preemptive pharmacogenomic result delivery for African-Americans in the inpatient setting across multiple institutions. The ACCOuNT Translational project is led by principal investigator Dr. Peter O’Donnell (University of Chicago) and site leads Drs. Kevin O’Leary (Northwestern University) and Edith Nutescu (University of Illinois at Chicago). Additionally, the ACCOuNT Translational project is supported by the Center for Personalized Therapeutics and other members of ACCOuNT.

“Precision Medicine for All”
The ACCOuNT Translational project is led by principal investigator Dr. Peter O’Donnell (University of Chicago) and site leads Drs. Kevin O’Leary (Northwestern University) and Edith Nutescu (University of Illinois at Chicago). Additionally, the ACCOuNT Translational project is supported by the Center for Personalized Therapeutics and other members of ACCOuNT. There are three specific aims of the translational project.
1. Incorporate race-specific SNPs and recommendations into the GPS.
2. Create a cohort of African-American patients receiving patient care using GPS.
3. Access race-specific recommendations via GPS in consortium institutions.
Self-identified African-American patients who enroll in the ACCOuNT Translational project provide a blood sample which is sent to the Center for Personalized Therapeutics’ CLIA-certified laboratory for genotyping. Genotyping results and clinical decision support are made available within the Genomic Prescribing System (GPS).
Providers who staff inpatient services (physicians, pharmacists, nurse practitioners, and physician assistants) are eligible to enroll for the ACCOuNT Translational project and access patient genotyping results and pharmacogenomic recommendations via the GPS. Each hospital admission at which a preemptively genotyped patient is treated by an enrolled provider is evaluated. Data captured includes provider use of the GPS, as well medications prescribed or administered during the course of the admission. The primary outcome of interest is frequency of GPS use by study providers.
ImPress Trial
The goal of the ImPreSS Trial is to develop a model of implementing point-of-care pharmacogenomic decision support in the perioperative care setting.
📌Image will be replaced by lab picture

To “ImPreSS”
Truong TM, Apfelbaum J, Shahul S, et al. The ImPreSS Trial: Implementation of Point-of-Care Pharmacogenomic Decision Support in Perioperative Care. Clin Pharmacol Ther. 2019;106(6):1179‐1183. doi:10.1002/cpt.1567
To learn more about the study, please access the link here Clinicaltrials.gov.

Overview
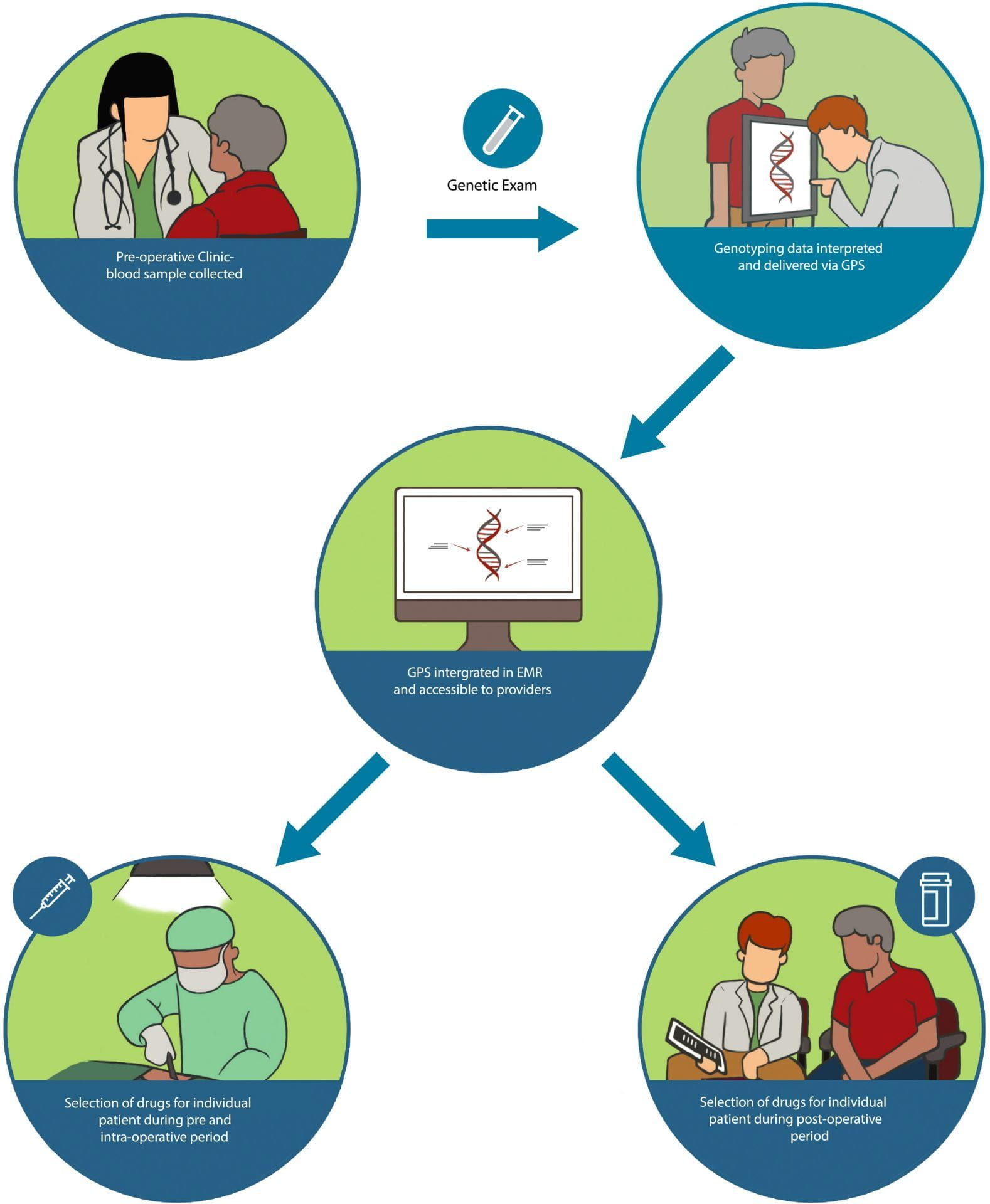
Anesthesiologists, critical care and pain management physicians, and associated providers (nurse anesthetists, physician assistants, and pharmacists) within the Department of Anesthesia and Critical Care (DACC) will be targeted for enrollment through a process of direct stakeholder engagement and informed consent. Adult patients with planned elective inpatient or outpatient surgeries will be eligible for the study. Enrolled patients provide a DNA sample during their pre-operative clinic at the Anesthesia Perioperative Medicine Clinic (APMC), which is sent to our CLIA-certified laboratory for genotyping, and results will be available before the patient’s surgical admission.
Patients’ genotyping results will be delivered to providers through our online web portal, the Genomic Prescribing System (GPS), which is currently integrated into our institutional electronic medical record. Genotyped patients will be prospectively randomized at the time of surgical admission to one of two treatment arms. In the pharmacogenomics arm, treating providers who care for the patient during the course of the surgical admission will have access to GPS and the patients’ pharmacogenomic information, whereas in the control arm, GPS and patient-specific pharmacogenomic information will be withheld from the providers, approximating current standard of care. We hope this study will advance our understanding of the use of genetic information in the perioperative setting.
PHOcUS Trial
Studying pharmacogenomic testing in Oncology care
Over 650,000 patients per year are treated with chemotherapy, with the majority reporting side effects. The goal of the PhOCus Trial is to determine whether delivering pharmacogenomic results to oncology providers will help reduce incidence of severe chemotherapy related side effects and toxicities.
📌Image will be replaced by lab picture

Overview
Oncology providers, including physicians, pharmacists, physician assistants, and nurses in the gastrointestinal (GI), head and neck, and breast clinics are enrolled and trained on the use of the Genomic Prescribing System (GPS).
Adult patients for whom chemotherapy with fluoropyrimidines (5-fluorouracil or capecitabine) and/or irinotecan is being considered are eligible. Enrolled patients will provide a DNA sample and will be randomized to one of two treatment arms. In the pharmacogenomics (PGx) arm, oncologists will have access to the GPS and patient-specific pharmacogenomic information, which may inform dosing decisions for chemotherapy as well as supportive care medications such as analgesics and antiemetics. In the control arm, care teams will not have access to pharmacogenomic information until 6 months after initiation of chemotherapy, and dosing will be according to standard of care. We hope this study will enhance our understanding of the use of genetic information in cancer care.
📌 Is there a quick link to perhaps the publication of this study?
Polymorphisms in the DPYD gene can result in enzyme deficiency leading to an increased risk of severe, sometimes fatal toxicities in up to 10% of patients.
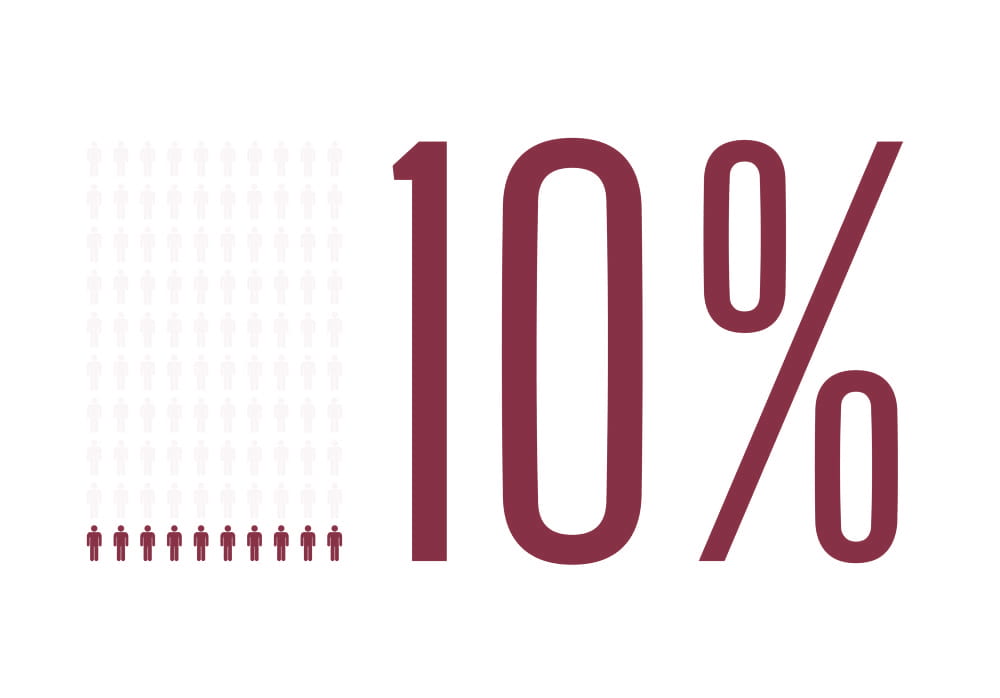
Compared to the historical cohort of *2A carriers who received full dosing, the risk of drug-induced death was reduced from 10% to 0% in *2A carriers receiving reduced dosing. Furthermore, the average total treatment cost was modeled as being modestly lower with screening, even when including the screening costs for the entire population.
Personalized Therapeutics Study (PTS)
Evaluating the Effectiveness of a Personalized Therapeutics Clinic on Drug-Drug and Drug-Gene Interactions,” or the “Personalized Therapeutics Study” for short, combines the use of pharmacogenomics with the establishment of the novel Personalized Therapeutics Clinic (PTC) at UChicago Medicine. Participants in this study ultimately have the opportunity to undergo genetic testing and have subsequent results analyzed to determine potentially-adverse drug-drug interactions (DDIs) and drug-gene interactions (DGIs). For those participants whose DNA is genetically tested, they also have the opportunity to attend a virtual appointment(s) with a healthcare provider from the PTC who will discuss with participants the genetic results and their implications.
As this is a clinical study, patients who consent to this study will be assigned to one of three different groups. The assignment to one of the following cohorts is completely random and computer generated:
- Regular care with your doctors (NO genotyping and NO PTC)
- Regular care AND a virtual appointment with the PTC (NO genotyping)
- Regular care AND DNA genotyping AND a virtual appointment with the PTC
Regardless of the group to which patients are assigned, they still have the benefit of being offered genetic testing and PTC virtual appointment(s) after 9 months of being enrolled in the study. Everyone’s genome is unique, and we are excited to present the patients of UChicago Medicine with this opportunity to better understand and govern their healthcare. By providing this study to our patients, we hope to better understand current prescribing practices, identify the convenience of a personalized therapeutics clinic, and ultimately decrease potentially-dangerous DDIs and DGIs.”
