ImPress Trial
The goal of the ImPreSS Trial is to develop a model of implementing point-of-care pharmacogenomic decision support in the perioperative care setting.
Our groundbreaking study aims to revolutionize the field of anesthesia and critical care by leveraging pharmacogenomics to enhance patient care. Anesthesiologists, critical care physicians, and associated providers will be enrolled in the study to assess the impact of personalized pharmaco-genomic information on patient outcomes.


To “ImPress”
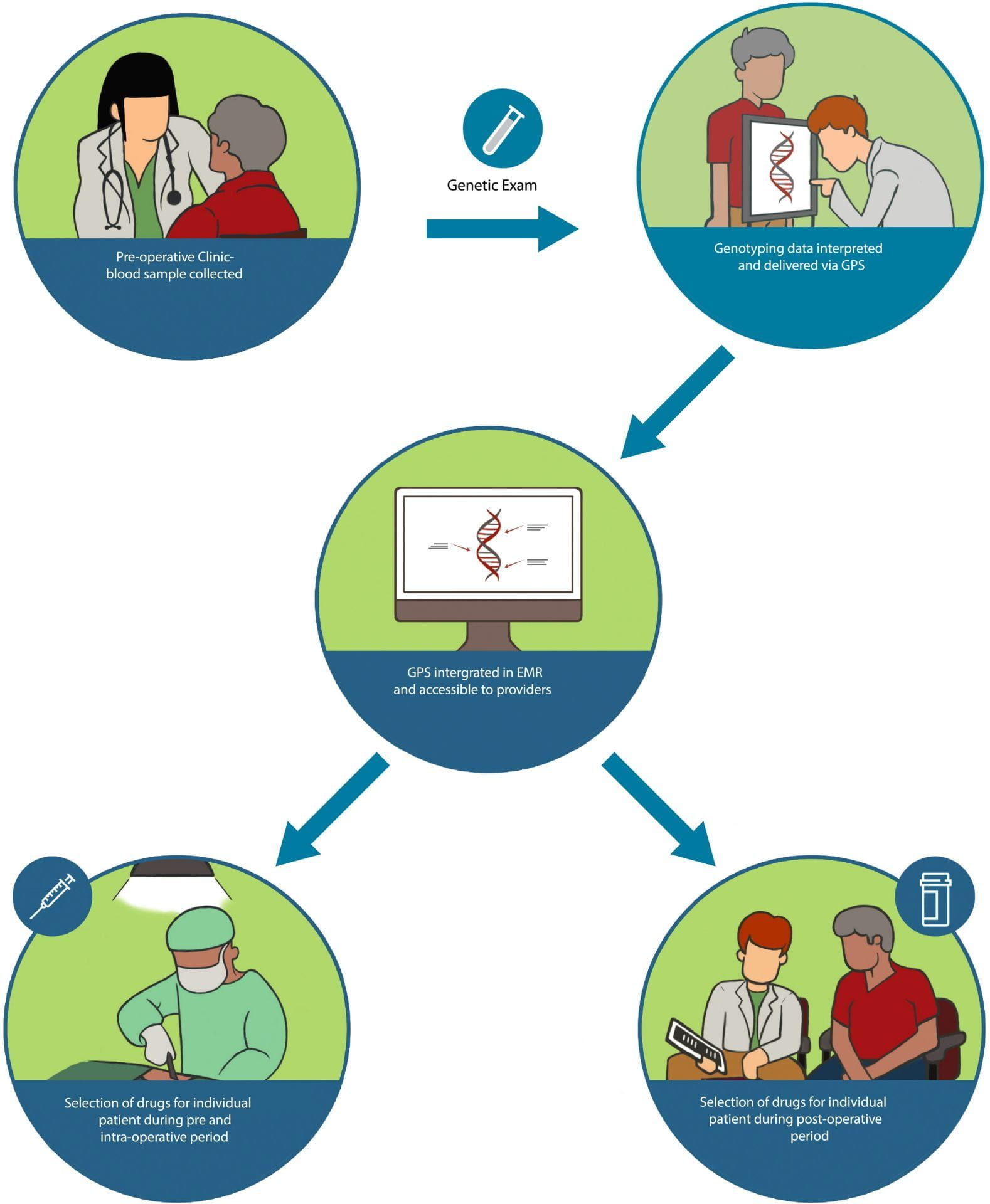
The ImPress Trial involves genotyping enrolled patients and delivering their genomic results through an online web portal integrated into the institutional electronic medical record. Providers in the pharmacogenomics arm will have access to this information during the patient’s surgical admission, allowing for tailored medication choices based on individual genetic profiles.
By tracking medication use and comparing the rate of high pharmacogenomic risk drugs between the two study arms, the primary outcome will provide valuable insights into the effectiveness of pharmacogenomics in guiding treatment decisions.
Read more

Revolutionizing Personalized Medicine in Anesthesia and Critical Care
1. Health Providers
3. Our Lab
2. Patients
4. Our Web Portal

Transforming anesthesia and critical care by integrating genomic information into clinical practice.
ImPress Trial has the potential to transform anesthesia and critical care by integrating genomic information into clinical practice, ultimately leading to improved patient care, enhanced pain management, and safer medication administration.
Point‐of‐Care Personalized Therapeutics Decision Support in Perioperative Care
Read on ClinicalTrials.gov
Genotypes
Sampling Technique
There will be an initial 6 month run-in period in which a small cohort of 100 will all have their pharmacogenomics results made available to participating treating providers. The purpose of the run-in period is to allow for process evaluation and refinement of pharmacogenomic information delivery. After the initial run-in period, genotyped patients will be prospectively randomized in a 1:1 ratio at the time of surgical admission to one of two treatment arms, with all medications administered/prescribed tracked for both arms.
Pharmacogenomics Arm
Control Arm
Genotyping technology
Data Analysis
Providers and patients will be asked to complete a survey after each surgical admission. Providers will also be asked to complete a questionnaire every 6 months during the study to assess general knowledge and understanding of, in addition to attitudes towards, pharmacogenomics. The primary outcome is to compare the rate of use of high pharmacogenomic risk drugs between the two groups.
Our institutional Data and Analytics Core will be utilized for a key sub-analysis to collect pain care quality data and assess pain management services for both arms, including pain assessment (patient reported pain scores using a 11-point numeric scale; determined from EMR), pain therapy administration, and rate of opioid-induced adverse drug events (ADEs). Reported ADEs will be reviewed to determine whether they were due to prescribed or administered medications during the admission, and whether these medication choices were potentially the result of pharmacogenomics related decision-support delivered to providers in the context of this study.
Clinical Trial
Empowering Clinical Critical Care through Genetics
The ImPress Trial is enrolling adults who are scheduled for either inpatient or outpatient elective surgical procedures at The University of Chicago. Genotyping results will be delivered to participating providers as patient-specific drug-gene clinical decision support summaries using a secured Web portal, the Genomic Prescribing System (GPS).
We invite you to seize this unparalleled opportunity to be at the vanguard of a transformative shift in surgical care. Align yourself with us as we usher in a new era of personalized medicine, ensuring that your surgical journey is thoughtfully guided by the intricacies of your genetic composition.
Transformative Surgical Care
By becoming a participant in the groundbreaking study, you will be presented with a unique opportunity to engage in a proactive approach to your healthcare. During your pre-operative visits, our dedicated team will provide you with comprehensive information about the trial, and subsequently collect a blood sample for preemptive genotyping. This advanced genetic analysis will unravel predictive insights into drug responses and toxicity risks, thereby ensuring the utmost safety and efficacy of your treatment regimen.





