Our Laboratory
Discover the cutting-edge world of the University of Chicago Advanced Technology (UCAT) Clinical Laboratory, where groundbreaking research meets revolutionary genotyping techniques.
Led by Dr. Yeo, this laboratory within the Department of Pathology collaborates closely with the Center for Personalized Therapeutics (CPT) to delve into the realm of clinical research. Get ready to embark on a journey through the realms of the 1200 Patients Project, ACCOuNT Project, ImPreSS Trial, and PhOCus Trial.
CLIA Laboratory
Empowering Pharmacogenomic Research through Advanced Genotyping
Each day, we strive to unravel the complexities of the human genetic code, revolutionizing the way we understand and approach personalized therapeutics.
Genotypes
Unveiling Genotypes
In conjunction with the CPT, the laboratory has developed and validated an extensive genotyping panel capable of analyzing numerous DNA variants associated with pharmacogenomics. Key genes, including CYP2D6, TPMT, CYP2C19, and NAT2, are subject to thorough scrutiny through the implementation of high-precision genotyping methods. Notably, the complex nature of the highly polymorphic CYP2D6 gene necessitates a specialized approach. Our lab utilizes the Hologic Invader assay for genotyping CYP2D6, with the Taqman assay complementing it for the analysis of CYP2D6 copy number variation.
Genotyping technology
Advanced Techniques
At the UCAT Clinical Laboratory, a range of advanced genotyping techniques is employed, showcasing the institution’s commitment to cutting-edge research practices. Our laboratory employs the QuantStudio 12K Flex Real-Time PCR System, in conjunction with OpenArray custom plates, which enables each genotyping run to yield comprehensive data on up to 480 variants per patient. Notably, due to the intricate nature of the highly polymorphic CYP2D6 gene, a distinct approach is warranted. For CYP2D6 genotyping, the Hologic Invader assay is utilized, allowing for the analysis of 28 star alleles giving physicians an unmatched comprehensive view of the CYP2D6 gene. Finally, The UCAT lab complements the 28 CYP2D6 star allele calls with Copy Number Variation to give a complete look at this very important drug metabolizing gene.
Projects
Assisting in Frontier Pharmacogenomic Projects
Our esteemed laboratory is proud to collaborate closely with the Center for Personalized Therapeutics (CPT), working hand in hand to revolutionize the field of pharmacogenomics. Through this collaboration, we have been instrumental in advancing groundbreaking projects such as the 1200 Patients Project, PhoCUS Trial, ImPress Trial, and ACCOuNT Trial. These endeavors aim to unlock the potential of personalized medicine, enhancing patient outcomes through the precise prediction of drug response.

Aid in Surgery
Implementing pharmacogenomic testing in anesthesia and perioperative medicine
ImPress Trial

Focus on precision
Studying the value of pharmacogenomic testing in oncology care
PhOCus Trial

Personalized care
Piloting the use of pharmacogenomics in routine medical care
1200 Patients Project

Minority Health
Examining pharmacogenomic implementation in the context of minority health disparities.
ACCOuNT Trial


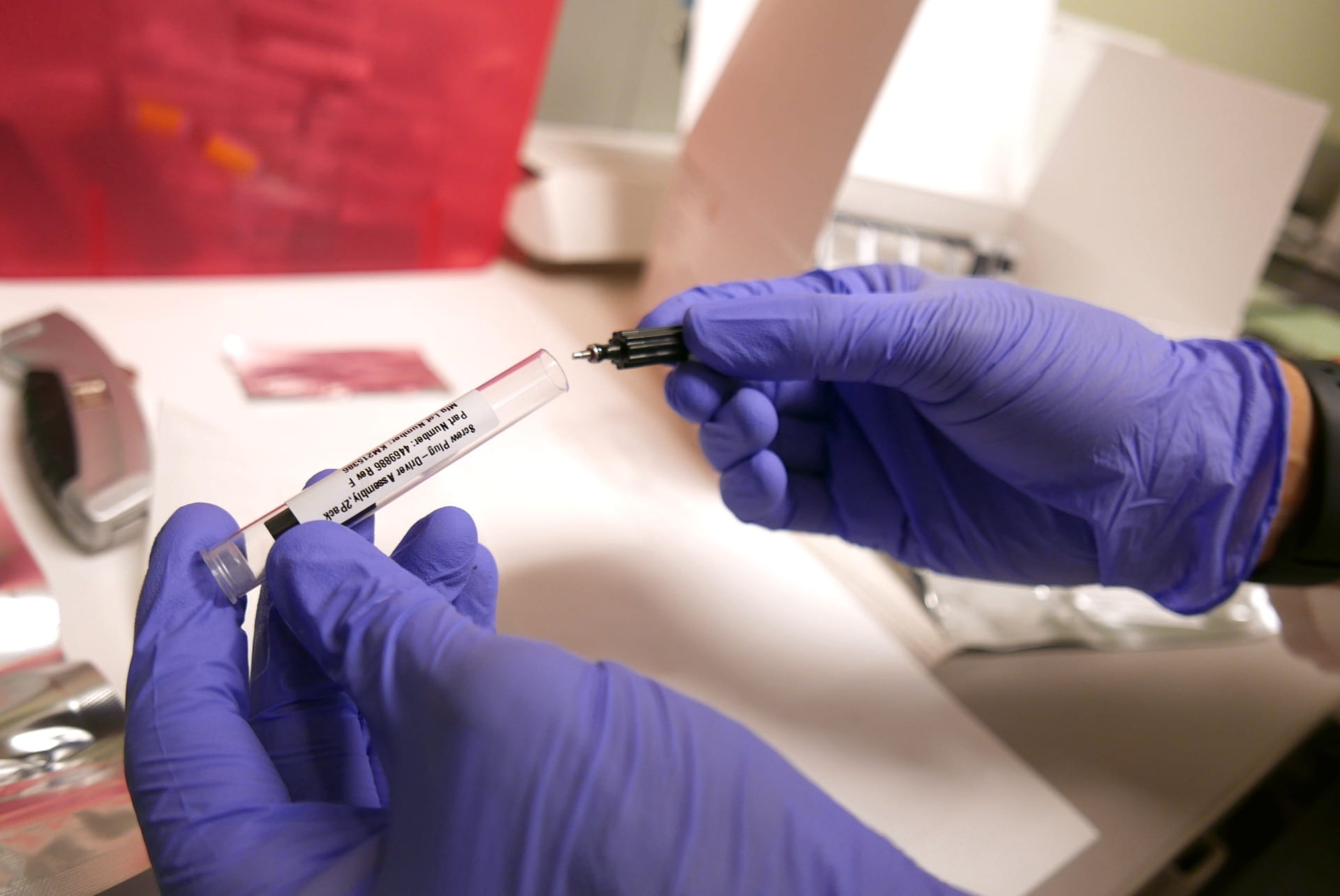

Unveiling Complex Genotypes: Our Story with CYP2D6
Genotyping cytochrome P450, family 2, subfamily D, polypeptide 6 (CYP2D6) is involved in the oxidative metabolism of approximately 20% of all clinically used medications. However, CYP2D6 is a challenge because of the high complexity of the locus.
Our validation of an extensive CYP2D6 assay panel, incorporating Invader and TaqMan Copy Number Variation, contributes significant insights into this complex gene. This research represents an exceptional collaboration between the UCAT Clinical Laboratory and the Center of Personalized Therapeutics (CPT), illuminating new pathways in the realm of genomics.
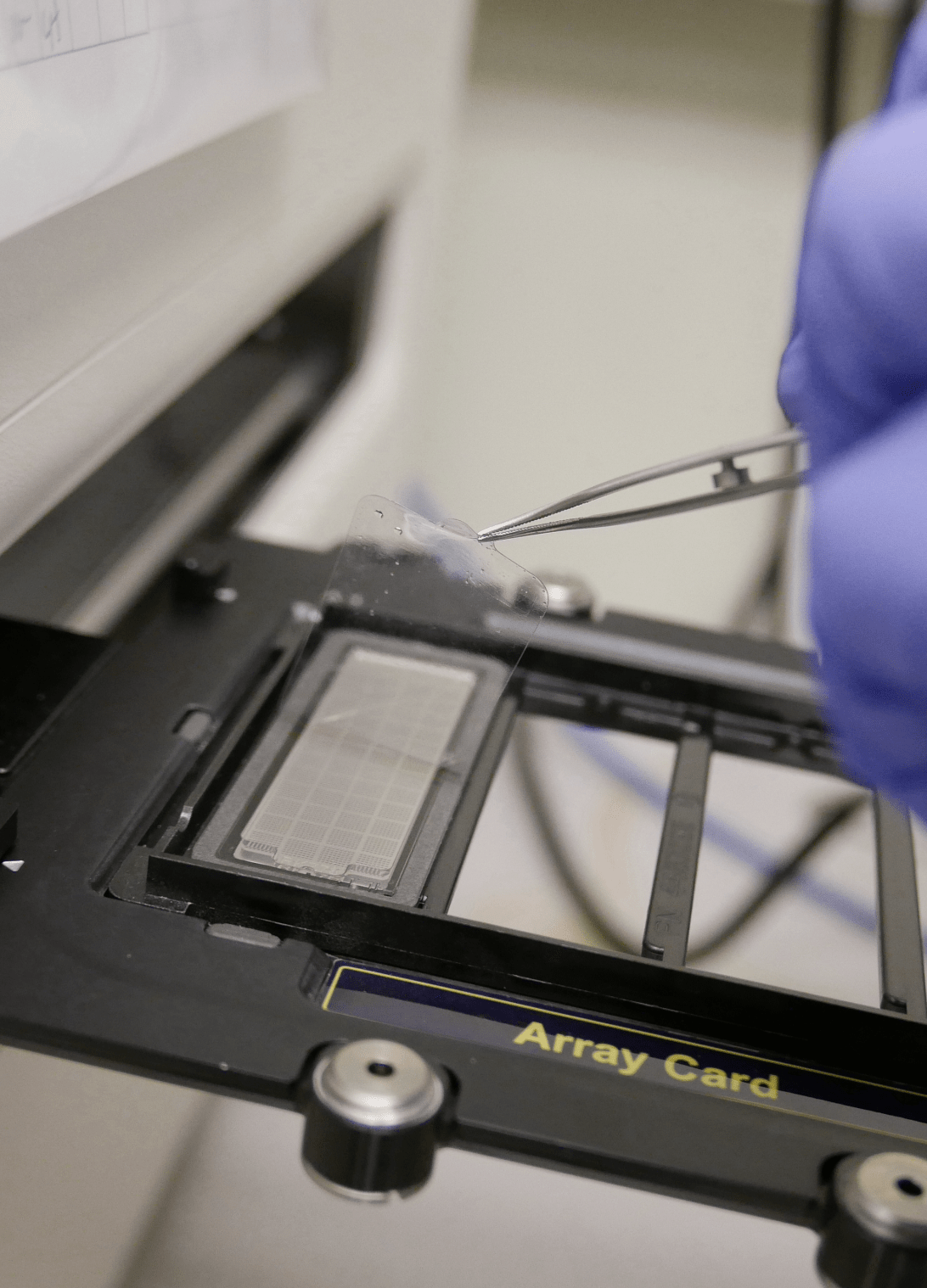
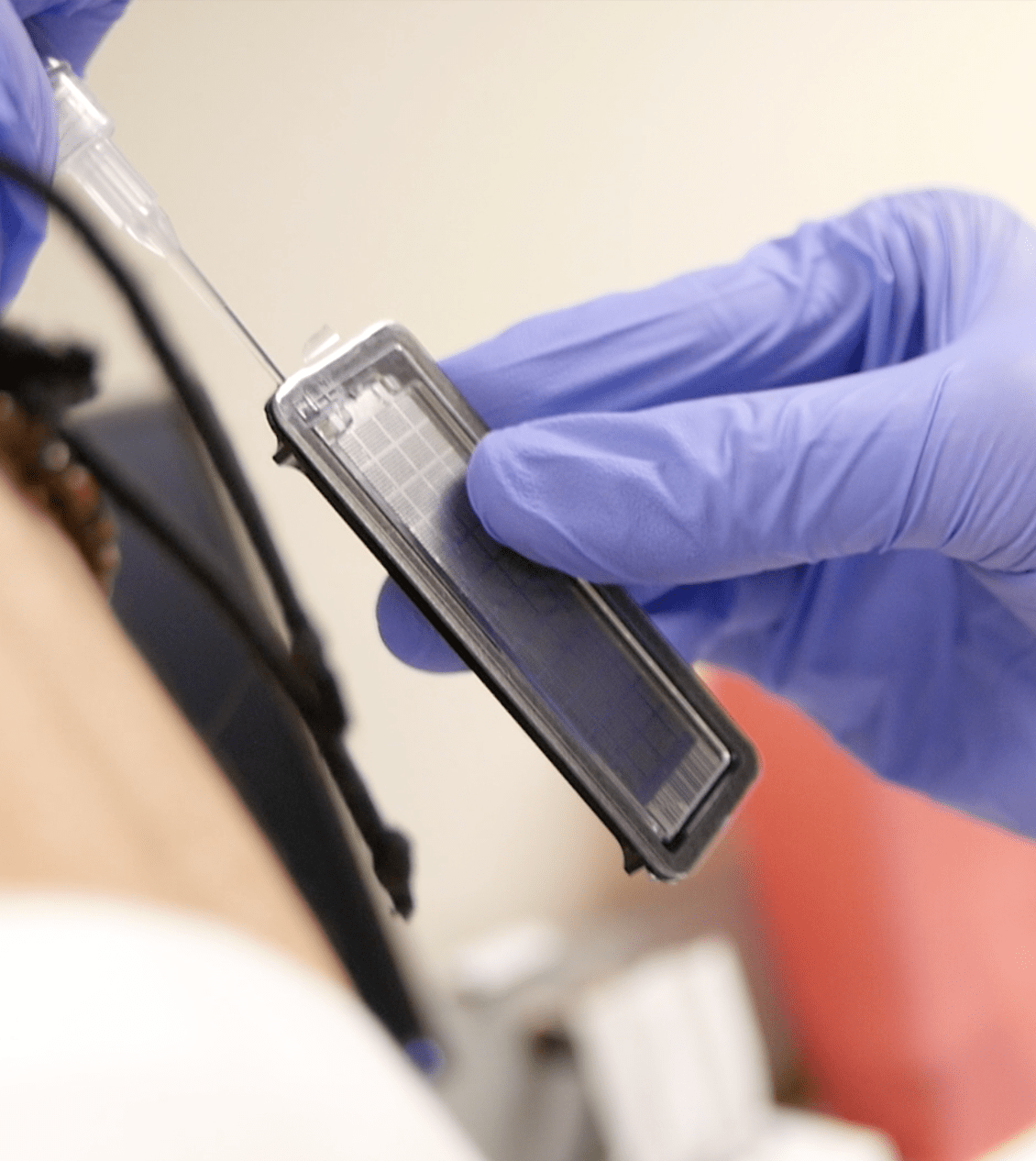
Publication
Robust Evaluation of Custom OpenArray® Pharmacogenomics Panel for Enhanced Clinical Decision-Making.
Pharmacogenomics has the potential to improve patient outcomes through predicting drug response. We designed and evaluated the analytical performance of a custom OpenArray® pharmacogenomics panel targeting 478 single-nucleotide variants (SNVs).
The study employed Coriell Institute cell line DNA samples and whole-blood DNA, comparing genotyping calls to reference methods. The panel demonstrated high concordance and reproducibility for most variants, with only a few exhibiting performance issues. DNA concentration of 50 ng/µL was recommended for optimal genotyping accuracy. The validated panel’s clinically actionable results are employed in pharmacogenomics-based clinical studies for informed decision-making.
Read Publication
With an unwavering commitment to personalized medicine, the UCAT Clinical Laboratory joins forces with the Center for Personalized Therapeutics (CPT) in harmonious collaboration and aid in ground-breaking projects such as the 1200 Patients Project, the ACCOuNT Project, the ImPreSS Trial, and the PhOCus Trial.
Precision and Reliability
Our laboratory holds Clinical Laboratory Improvement Amendments (CLIA) certification and College of American Pathologists (CAP) accreditation, assuring adherence to rigorous quality standards. Together, we embark on a quest to prepare and analyze genotype specimens that lay the foundation for groundbreaking pharmacology research initiatives.




Lab Members
Our laboratory is directed by Dr. Yeo. We work closely with the Center for Personalized Therapeutics (CPT) to prepare and analyze specimens for clinical research.
K-T Jerry Yeo,PhD, DABCC
Principle investigator
Professor and Director, Clinical Pharmacogenomics Laboratory
Department of Pathology.
Director, CLIA Genotyping Laboratory, Center for Personalized Therapeutics, The University of Chicago

Larry House
Genomics Laboratory Scientist
Department of Medicine, Section of Hematology/Oncology
CLIA Genotyping Laboratory,
Center for Personalized Therapeutics
The University of Chicago

David George
Clinical Genomics Scientist
Department of Pathology, CLIA Genotyping Laboratory, Center for Personalized Therapeutics, The University of Chicago
Join us on this remarkable journey as we continue to revolutionize clinical research through meticulous genotyping techniques.
Phone
(773)-753-1200
Open Hours
Mon – Fri: 9:00 AM – 4PM
Address
5841 South Maryland Avenue, MC 2115


